Mentioned in Video:
- 🧬🧪 Talking Genomics with ARK Invest's Simon Barnett: https://www.youtube.com/watch?v=TO8_NdadMy8
- 🧬 ARKG | MASSIVE Breakthrough for ARK Invest's Genomics Stocks: https://www.youtube.com/watch?v=WCCv0H_fgU8
- Ginkgo Bioworks SPAC Deal (SRNG on CNBC): https://www.youtube.com/watch?v=BfA2LET0W4c
- Ginkgo Bioworks Investor Day Presentation: https://www.youtube.com/watch?v=vSOUxOIzkbg
- Ginkgo Bioworks Investor Relations: https://investors.ginkgobioworks.com/overview/default.aspx
- Support the channel and get extra member-only benefits by joining us on Patreon: https://www.patreon.com/tickersymbolyou
🧬 #CathieWood may have just found the next Amazon stock! #ARKInvest has been loading up on #GinkgoBioworks (formerly #SRNG stock, now #DNA stock) in #ARKK and #ARKG (@ARK Invest‘s #genomics fund). This company is trying to be the Amazon Web Services (AMZN stock) of synthetic biology. In this episode, I go over what the heck that actually means and whether or not SRNG is one of the best stocks to buy now as a result.
Video Transcript:
[00:00:00.610]
It could not be closer to how we think about the future of investing in general, which is the multiplicative effects of all of these different exponential cost curves colliding in such a way that you get totally freakish and alien output.
[00:00:16.690]
Labgrown CBD, plantbased meat and bringing back the perfume of an extinct flower.
[00:00:21.970]
One CNBC Disruptor 50 company is making all that a reality with what they call made to order microbes.
[00:00:27.970]
Sometimes investing in the future is awesome. Over the last few weeks, I've been sharing deep dives into some of my favorite ARK Invest holdings that got oversold during the recent rotation from growth stocks into value stocks and cyclicals. This time I'd like to shake things up a bit and talk about Ginkgo Bioworks, a synthetic biology company that's trying to become the Amazon Webservices of programming cells. So in this episode, I've pulled together a bunch of clips to help explain what the heck that actually means, what kinds of technologies are converging to make it possible.
[00:00:59.230]
And of course, how Ginkgo Bioworks makes money, so we can decide whether or not this is actually a good investment. Let's splice right into it. The first thing you should know is that Ginkgo Bioworks is combining with a special purpose acquisition company (or SPAC) called Soaring Eagle Acquisition Corp, ticker symbol SRNG. If that name sounds familiar, it's because DraftKings did a SPAC merger with Diamond Eagle Acquisition Corp. And Skillz did one with Flying Eagle Acquisition Corp. Soaring Eagle is the latest SPAC formed by the same team, and ARK Invest currently holds all three of those stocks. DraftKings, Skillz
[00:01:34.210]
and now Ginkgo Bioworks. In fact, ARK Invest helped fund the PIPE for this SPAC. PIPE stands for private investment in public equity, meaning ARK Invest bought in as early as they possibly could. So did Bill Gates, by the way. In addition, ARK Invest started buying SRNG stock in early August, and today Cathie Wood has $170,000,000 of Ginkgo Bioworks stock, making it their 57th biggest position overall out of about 175. I expect that number to keep growing over time, especially once Ginkgo's SPAC merger goes through later this year and they start trading under the ticker symbol DNA.
[00:02:09.610]
Talk about a great ticker. So what does ARK Invest actually see in this company? I'm not an expert in synthetic biology, so to help answer that question, let's turn to Simon Barnett. Simon is one of ARK Invest's analysts on the Genomic Revolution, where he focuses on next generation DNA sequencing, bioinformatics and synthetic biology. Here he is speaking about Ginkgo Bioworks in my exclusive interview with him earlier this summer.
[00:02:33.550]
There's a company called Ginkgo Bioworks. It's one of the largest synthetic biology companies now, through its financing. They have this series of literally like biological factories. They're called Bioworks or Foundries. And there's five of them. I think now there might be more by the time we finished this, but they're all based in Cambridge, up in Boston and each of these things is like an amazing interplay between some of the most sophisticated and experimental life science tools, things that are looking at single cell biology in a massively parallel fashion, and the robots that are doing it are all super low latency because the software and the code base is being updated so quickly that these things are learning by the end of every workday, they might be behaving slightly differently than they did at the beginning of the workday.
[00:03:21.190]
It's really amazing to see that interplay between different… I mean, it could not be closer to how we think about the future of investing in general, which is the multiplicative effects of all of these different exponential cost curves colliding in such a way that you get totally freakish and alien output.
[00:03:41.590]
Speaking with Simon really helped me understand what types of technologies and trends to look for in these types of companies. And every time I listen to that interview, I end up learning something new. If you want to check it out, I'll leave a link to that interview in the top right hand corner of your screen right now. And in the description below as well. Many of the companies inside ARK Invest's funds are riding down multiple cost curves at the same time. In the case of Ginkgo Bioworks, those cost curves would be industrial robotics, artificial intelligence training, DNA sequencing, as well as economies of scale.
[00:04:13.810]
And the freakish and alien outputs that Simon mentioned are the specialty cells that Ginkgo is able to deliver at a fraction of the cost, I think. But unlike many other companies that focus on one thing, Ginkgo Bioworks is building a horizontal platform to program cells for every type of application, from pharma and biotech to food and agriculture and even consumer products. Before I dive into their platform and how they're able to do this, here's a quick clip of Jason Kelly, the CEO of Ginko Bioworks,
[00:04:42.370]
giving a few examples of what the company actually makes.
[00:04:45.790]
Labgrown CBD, plantbased meat and bringing back the perfume of an extinct flower.
[00:04:51.070]
One CNBC Disruptor 50 company is making all that a reality with what they call “made to order microbes”. With me now is Jason Kelly. He's the CEO of Ginkgo Bioworks, number 19 on our list this year. Welcome and congrats.
[00:05:02.890]
Yeah. Thanks, Kelly. Thanks for having me.
[00:05:04.150]
So first of all, because beyond meat and Impossible burger are so in the news today…
[00:05:07.630]
A little bit in the news. Yeah.
[00:05:08.590]
I know they're not direct customers, correct. But you are creating what exactly for this nascent industry.
[00:05:13.990]
Right. So if you take a bite out of that impossible Whopper at Burger King, it's going to bleed. Right. And what's going on there is they put hemoglobin, which is what makes blood red, which you don't really find at high levels in plants into the burger. So where does it come from? Well, they took Brewer's yeast like they used to use to make beer and they program it like you'd program a computer.
[00:05:33.310]
You can program yeast?
[00:05:34.570]
You can. Yeah.
[00:05:35.110]
You can actually program any cell. Right. So if you look out in nature and you look inside, any plant, animal or insect in the cell will be code in the form of DNA.
[00:05:44.110]
Wow.
[00:05:44.530]
And it's ATCG, not zeros and ones, but it's a lot like programming a computer.
[00:05:48.130]
So basically, you're programming yeast to make heme.
[00:05:51.370]
Wow.
[00:05:52.150]
Milk, protein or whatever.
[00:05:53.530]
Or CBD. Is that right? Exactly. That's a partnership with Kronos.
[00:05:56.770]
Yeah. Kronos is a Canadian cannabis company. And so the way you get CBD for CBD oil today is you extract it from a cannabis plant. You grow this whole plant with a flower and all this, and you take out this little teeny bit of CBD oil or THC or these other cannabinoids. So what we'll do is we'll read the DNA of that plant, find the code for the CBD, move it into a yeast or bacteria, and then you brew it up and you get CBD oil. No plant.
[00:06:19.570]
Right.
[00:06:19.690]
So one of the things you learn quickly about biology is there's enormous reuse of that DNA code across biology. So it's kind of surprising you can take, like, the code from a cannabis plant and put it in yeast, and it works. And the reason is we all share the same stuff.
[00:06:32.290]
So the big idea here is that Ginkgo Bioworks can help businesses across many different industries and market sectors move to a much cheaper manufacturing process for the materials that are the most time consuming or expensive to make, effectively removing bottlenecks in their production. The list of end markets their platform can serve is massive, and they're able to work in all these different markets because their platform focuses on the thing they all have in common: reading and writing the genetic code of the underlying cells. That means Ginkgo's customers don't need to build their own labs and teams of bio and chemical engineers and can instead focus on their own core businesses.
[00:07:08.530]
Just like when companies choose to build a store on Shopify instead of building a custom website or building applications on Amazon Web Services instead of building their own infrastructure. That's what Ginkgo Bioworks's platform enables in the genomics space. That platform really has two parts, their code base of cells, enzymes and genetic programs that they use to jumpstart new projects and Ginkgo's automated foundries. Let's start with their code base. Here's a quick clip of Barry Canton, Ginkgo's chief technology officer, explaining the basics.
[00:07:38.470]
One of the first questions that we always get from folks is what is our foundry and what do we do in there? And that's a very reasonable question, because foundries and this concept of programming biology is foreign to almost everybody. For us, it typically starts with an interaction with a potential partner or customer, where we jointly develop the concept of a sell program that will help that customer make a new product or a better version of an existing product. Through those conversations, we'll develop a specification for the cell program that we're going to build with them.
[00:08:15.670]
Once we've reached that agreed upon specification, the work is turned over to our cell designers, who will refine and develop the concept and the specification of how we're going to make that cell program. They'll continue to develop that using code base from our collection as well as nature's codebase, the cells and genetic assets that are out in nature, and our designers will bring those different pieces of code base together to develop a detailed design. We put all of our best learning accumulated over many cell programs into those early designs.
[00:08:53.590]
Once we have those detailed designs that are specified at the level of DNA sequences, all in a computational manner, those designs are handed over to our DNA synthesis and our build teams. Their task is to take those conceptual and computational designs and turn them into reality in the lab.
[00:09:14.290]
So Ginkgo Bioworks adds a lot of value for their customers at this step by helping build out the right specifications for the cells themselves and by applying existing genetic codes to new projects. Because Ginkgo Bioworks operates in many different verticals, they can take lessons learned from one program and apply it to others as well as continue to build out that library of genetic codes, to help ramp up even more new customers faster and faster over time. As their CEO Jason Kelly, said, there's a lot of reuse across different programs.
[00:09:44.590]
For example, the same yeast used to generate proteins for food can produce proteins for personal care products as well. So when Ginkgo learns how to manipulate yeast for one customer, they learn how to do it for a wide variety of applications and future potential customers as well. This forms a virtuous cycle where the more clients Ginkgo Bioworks can get, the more upfront value they can provide to each future client. Each new program lowers the overall cost of running the Foundry in the future, which means lower cost per program, which raises demand, resulting in more programs, which increases the amount of code in the codebase and on and on it goes.
[00:10:20.170]
That code base is a long term competitive advantage for Ginkgo Bioworks, with over 440,000,000 proprietary gene sequences acquired so far. I also love this quote by Patrick Boyle, Ginkgo's head of code base. “Ginkgo will organize the world's biological code and make it useful.” That sounds a lot like what Google does with information. The other side of their platform is the Foundry, which is scaling at roughly 3X per year. The new technologies they're working with are going to improve the capacity of their foundries by another factor of ten in the coming years.
[00:10:51.910]
They even give us their own version of Wright's Law, which they call Knight's Law. I'm guessing after Tom Knight, one of their founders. Every year, Ginkgo's foundries reduce the cost to genetically engineer a cell by 50% and threefold the number of designs they can test. How do you compete with that kind of scale and cost decline? Here's another quick clip of Barry Canton explaining what's inside these foundries.
[00:11:15.550]
For our Foundry to be efficient, what we've had to do is start with a conventional lab and then bring a lot of concepts in from manufacturing, from operations research in order to build scalable high throughput automated processes that allow us to more quickly and effectively program cells for our partners. And so what you will see in our Foundry is not the typical row after row of benches with a scientist working at each bench, you will see some of that in our foundries. But what you will see more and more of is sophisticated instrumentation, robotics, liquid handling instruments, and a wide array of sophisticated and complex machinery and instrumentation that we use to amplify and multiply what our scientists are able to do in our Foundry.
[00:12:15.910]
We bring the best in robotic and software automation, together with the unique skills and insights that humans have in order to be able to program cells more effectively, with higher probability of success than would ordinarily be possible.
[00:12:32.890]
I'm thinking of Ginkgo Bioworks' foundries as kind of like Tesla's gigafactories, but with many different types of cells being the final product. My point here is that they're both on the bleeding edge of automation in their respective industries. Unlike the automotive industry, where manufacturing has been highly automated for decades, the bioengineering industry is still very manual and highly specialized. That gives Ginkgo Bioworks a real advantage as long as they continue to push the limits in terms of automation, scale and the throughput of these foundries.
[00:13:03.010]
Ok.
[00:13:03.550]
So this is what we've been building towards. Now that we know what Ginkgo Bioworks does and a little more about how their foundries and codebase work, let's decide whether this company is worth investing in. Here's one last clip of Jason Kelly on CNBC walking everyone through how everything we've covered so far comes together.
[00:13:21.010]
So Ginkgo's businesses, we program cells kind of like you'd program a computer. So if you had an MRNA vaccine, that's a piece of RNA code. It's ATCs and Gs, not zeros and ones, but your cells read it, they make a little protein. They turn your immune system on. Well, what Ginkgo has here in Boston is about 200,000 sqft of facility to read and write that DNA code. And we work with companies, we have a 100 million dollar joint venture with Bear Crop Science. We work with Roche in Antibiotics. We did a project with Moderna last March around the vaccine supply chain side. We're like an AWS for programming biology.
[00:13:58.210]
It's an interesting metaphor, so I guess really, instead of custom software that you're creating for companies, it's really these custom genomes, if you will. How does the actual business model work? How are you making money on this?
[00:14:09.250]
Yeah. So what we do is we really have two ways of bringing revenue. One is while we're doing a project for a customer, they pay us on a usage basis. Think like using an Amazon datacenter. You pay them for compute. You pay us when our robots move to program that cell for you. That's the first way and then second, when we're done after, say, a one to three year project, we do a value share. Think like Apple App Store, a reach in to revenue either through a royalty or in lieu of a royalty equity in the company.
[00:14:37.330]
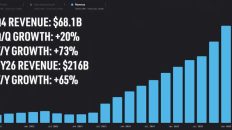
And so those are the two big revenue streams for us. And today the majority of our revenues, we're projecting 150,000,000 revenue in 2021, up 95% from last year. That's largely in the Foundry revenue coming from usage. But in the future, there's an enormous opportunity for bioengineered cells. McKinsey projects two to $4 trillion for sale applications in the future. I think ultimately it'll be bigger than apps and computers.
[00:15:01.210]
Jason, you mentioned 150 million in revenue and your Foundry billable revenue for 2021 at about 100 million. You see that increasing tenfold over the next four years. Let's call it 1.1 billion by 2025. What gives you the confidence that you can ramp revenues to that extent?
[00:15:19.210]
Yes, so the great thing is the kind of work we do is actually already done out at biotech companies today. It's just done by people with my background. I did a PhD at MIT in Bioengineering. You want to get one of those? It's about five years of moving liquids around a lab bench by hand. We take that work and we move it on to robotics, drop the cost with scale. Well, today in that industry last year, there's a nice paper report, spent about $33 billion on that kind of work.
[00:15:45.190]
And so what we're doing is migrating that. Think like going from on prem servers and IT to cloud. There is that existing spend. So out of that 33 billion, as you're mentioning, only 100 is coming to us right now. So there's a real opportunity for growth there. It's just the first inning.
[00:16:03.070]
So Ginkgo Bioworks makes money in two ways: when the robots do the actual work and then through a revenue sharing model or in equity in the company afterward. Today, most of their revenues are coming from the usage of the robots in their Foundry, which has doubled year over year, and Jason thinks it will ten X in the next four years. That's highly predictable revenue based only on the work the robots do, regardless of the program's ultimate success. The bigger piece of their overall revenue will come from royalties or equity the company will be getting from their clients.
[00:16:33.790]
In my opinion, this basically means when you invest in Ginkgo, you're also investing in the clients they take on, making this company almost like a portfolio of synthetic biology products and companies of its own. They already have 54 such programs that they'll be getting royalties from or equity in in the future, worth half a billion dollars today. They can accelerate adoption and keep growing this portfolio in a couple of ways by tapping into new markets and by growing existing customer accounts by launching new programs with them.
[00:17:03.070]
Their existing 2021 sales pipeline is indeed across a wide variety of industries and verticals, which is great to see.
[00:17:09.790]
That tells me that they're well past trying to prove out their business model in a given industry and they really know how to generalize their solutions. That's really important. Over the next five years they expect to go from 23 cell programs to 508, a compound annual growth rate of over 110%. My guess is Ginkgo Bioworks will end up being really sticky, meaning their clients will have a hard time dropping them because they'd probably end up having to stack several other companies together to replace Ginkgo if they didn't want to build out those capabilities in house.
[00:17:41.050]
Either way, it's a huge time and money sync, and they still wouldn't benefit from the same cost declines that Ginkgo is already enjoying. Overall, they're expecting their Foundry revenue to increase by over 50% in 2021 and by over 75% every year after through 2025. Likewise, they expect their number of new programs to grow by triple digits, or close to it, every year for the next five years. So huge growth potential. I'll leave a link to this investor presentation as well as their Investor Day videos in the description below.
[00:18:10.990]
Comment below with your thoughts on Ginkgo Bioworks. What kind of markets do you see them getting into next? Does this company excite you or are synthetic biology companies not really up your alley? Either way, I hope this episode helped you understand a little more about why ARK Invest has been loading up on Ginkgo Bioworks. What kinds of products they create at scale with their Foundry and code base as well as their financial present and future. If it did, let me know by investing in the like button and subscribing to the channel with all notifications turned on. That's a great way to invest in the channel that invests in you. Until next time.
[00:18:46.030]
This is Ticker Symbol: You. My name is Alex, reminding you that the best investment you can make is in you.
If you want to comment on this, please do so on the YouTube Video Here